January is National Cervical Cancer Awareness Month
In our previous cervical cancer awareness post, we covered screening and diagnosis:
https://bloom-obgyn.com/january-is-national-cervical-cancer-awareness-month-3/
And now…..
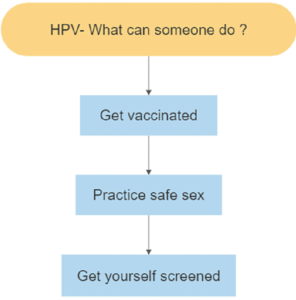
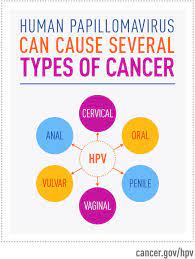
CERVICAL CANCER: PREVENTION
Human Papillomavirus (HPV) vaccine
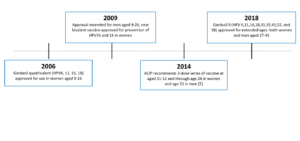
The first HPV vaccine was approved by the U.S. Food and Drug Administration or FDA in 2006. This vaccine – Gardasil 4 – protects against 4 strains of HPV. The Advisory Committee on Immunization Practices (ACIP) provides advice and guidance to the Director of the Centers for Disease Control and Prevention (CDC) regarding use of vaccines for control of vaccine-preventable diseases and ACIP recommended Gardasil 4 for girls and women aged 9 – 26 years old.
In 2009, the FDA approved another vaccine, Cervarix. This vaccine protects against two high-risk strains of HPV. Also in 2009, ACIP extended its HPV vaccine recommendation to include boys and men aged 9 – 26 years old.
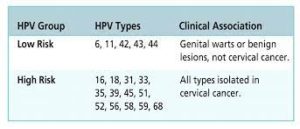
Gardasil 9 protects against 9 strains of HPV
Low-risk strains: 6, 11
High-risk stains: 16, 18, 31, 33, 45, 52, and 58
Gardasil 9, our current HPV vaccine, was approved in 2014. It protects against nine HPV strains, including high-risk types associated with cancer and low-risk types associated with genital warts. While still available worldwide, Gardasil 4 and Cervarix were discontinued in the United States in 2014.
In 2018, Gardasil 9 was approved for use in both women and men ages 27 – 45 years old
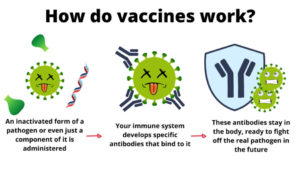
HPV vaccines use a protein from the shell of certain HPV strains. They do not contain viral RNA or DNA and they cannot cause disease. These viral proteins stimulate an antibody response so your immune system is primed to recognize HPV. If you are exposed, these antibodies work to prevent infection. HPV vaccines targeting high-risk HPV strains have been shown to be effective in preventing cancer in women and men. Additionally, Gardasil 4 and Gardasil 9 offer protection from several low-risk HPV strains that are associated with genital warts.
What is the HPV vaccine schedule?
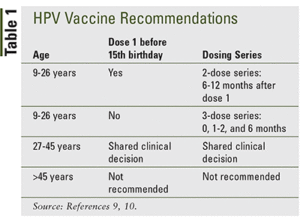
Ideally, vaccines should be administered before any exposure to HPV through sexual contact and initial HPV vaccination can start as early as age 9 years old. Typically the series is started by a Pediatrician in both girls and boys ages 11 – 12 years old. If vaccination is started prior to the 15th birthday, then we use a 2-dose series. If vaccination is started after a 15th birthday, then the HPV vaccine schedule is a 3-dose series and can be given up to age 45 years old.
Catch-up vaccination (ie, receiving the remaining doses in a series if someone falls off track with their timeline and is not adequately vaccinated) is recommended for all persons through age 26 years old. Catch-up HPV vaccination is not recommended for adults older than age 26 years old since the health benefits of vaccination in this age range is minimal.
HPV vaccines are not licensed for use in persons older than age 45 years old and, for those age 27 – 45 years old who have not previously received the vaccine, it’s recommended to discuss with your provider the potential benefits based on your risk of exposure
What are the side effects of the HPV vaccine?
While many people who get the HPV vaccine have no side effects at all, some report having very mild side effects. The most common side effects of the HPV vaccine are include:
- Pain, redness, or swelling in the arm where the shot was given
- Fever
- Dizziness or fainting (fainting after any vaccine, including HPV vaccine, is more common among adolescents than others)
- Headache or feeling tired
- Nausea
- Muscle or joint pain
To prevent fainting and injuries from fainting, adolescents should be seated or lying down during vaccination and for 15 minutes after getting the shot.
Very rarely, severe (anaphylactic) allergic reactions might occur after vaccination.